

HuidaGene Therapeutics Announces Eleven Presentations at the European Society of Gene and Cell Therapy (ESGCT) 2024 Annual Congress
SHANGHAI and CLINTON (NJ), October 08, 2024—HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company for genome medicines, today announced that eleven presentations (one oral and ten poster presentations) will be featured at the European Society of Gene and Cell Therapy (ESGCT) 31st Annual Congress, which is held from October 22-25, 2024, in Rome, Italy.
“The number of acceptances for presentation at this year’s ESGCT congress underscores our commitment to remain at the forefront of genome medicine to develop a new generation of therapies overcoming several difficult-to-treat diseases, including AMD, MECP2 duplication syndrome, and Alzheimer’s disease using hfCas13 RNA-editor, and ALS, Huntington, Angelman syndrome, and DMD using our novel hfCas12Max DNA-editor,” stated Alvin Luk, Ph.D., M.B.A., C.C.R.A., Co-Founder and Chief Executive Officer of HuidaGene. “The collective data presented provide further preclinical and clinical validation of our platforms and reflect the progress we continue to make, particularly in ophthalmology and CNS programs. I look forward to orally delivering our HG004 using a better viral vector of RPE tropism for inherited retinal diseases to reduce the total vector dose and injection volume, mitigating the safety risks related to surgical procedure and AAV-mediated toxicity.”

“Besides the robust pipeline, the Company will also present the superior editing efficiency of our hfCas12Max and our engineered AAV vector carrying mRNA (RAAV) to transiently express Cas proteins,” said Linyu Shi, Ph.D., Co-Founder and Chief Scientific Officer of HuidaGene. “We demonstrated that our proprietary CRISPR-based HG-PRECISE® platform can improve editing efficiency and reduce off-target effects, turning revolutionary science into transformational medicines for patients with life-threatening diseases worldwide. We look forward to sharing data at ESGCT, highlighting our innovative pipeline and “all-in-one” AAV gene-editing toolbox.”
The oral and poster presentations from HuidaGene at the 31st ESGCT Annual Meeting include:
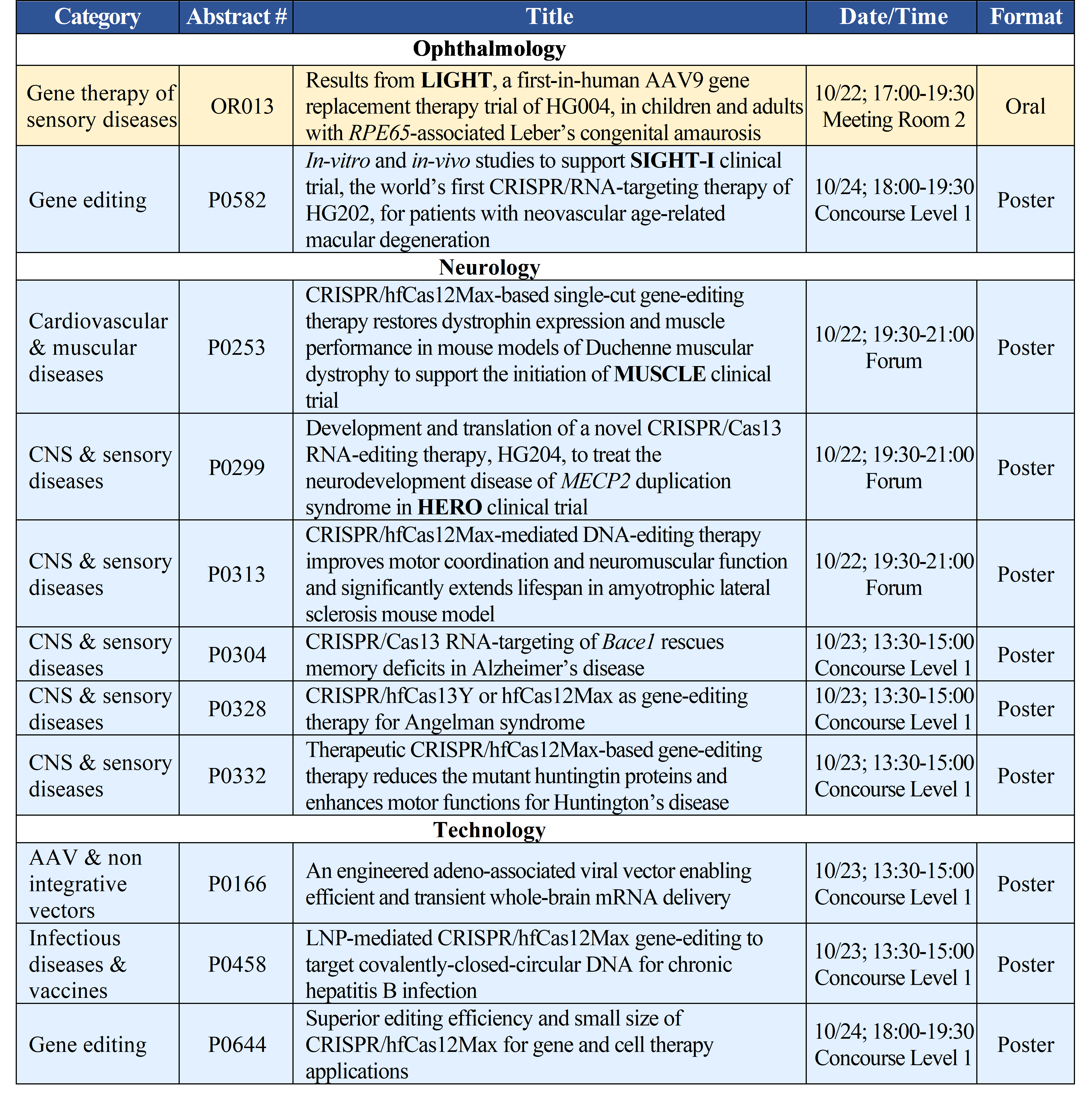
Accepted abstracts and the complete preliminary program are available on the ESGCT website (https://www.esgctcongress.com/programme).
About HuidaGene
HuidaGene utilizes its proprietary CRISPR-based HG-PRECISE® platform to develop potentially curative genome medicine. The Company is advancing clinical programs, including trials of HG004 (granted ODD & RPDD by FDA) ‘LIGHT’ trial (NCT06088992) and Phase 1/2 international, master-protocol ‘STAR’ clinical trial (NCT05906953) in RPE65-associated retinal disease, HG202 RNA-editing therapy ‘SIGHT-I’ first-in-human trial (NCT06031727) and Phase 1 ‘BRIGHT’ clinical trial (NCT06623279) for nAMD, HG204 RNA-editing therapy (granted ODD & RPDD by FDA and ODD by EMA) ‘HERO’ trial (NCT06615206) for MECP2 duplication syndrome, and HG302 DNA-editing therapy (granted ODD & RPDD by FDA) first-in-human ‘MUSCLE’ trial (NCT06594094) for DMD. The preclinical programs include HG303 DNA-editing for ALS and CRISPR RNA-editing therapy for Alzheimer’s. With an extensive intellectual property portfolio, HuidaGene is a leader in genome medicines for neurology and ophthalmology. Learn more at huidagene.com or on LinkedIn.
-
Previous
HuidaGene Therapeutics Appoints TJ Cradick, PhD as Chief Technology Officer to Lead Delivery Science and Genome Editing Innovations
-
Next
HuidaGene Presents Late-Breaking Data at World Muscle Society 2024 Demonstrating HG302 CRISPR/hfCas12Max DNA-Editing Therapy for Duchenne Muscular Dystrophy to Support M.U.S.C.L.E. Clinical Trial
recommendations
-
Dec 12,2024
HuidaGene Therapeutics Initiates M.U.S.C.L.E. Clinical Trial of HG302 for Duchenne Muscular Dystrophy and Completes First Patient Dosed
-
Apr 11,2025
HuidaGene at CRISPR MEDiCiNE 2025: A Celebration of Progress, Promise, and Patients
-
Nov 04,2024
HuidaGene Therapeutics Receives the First-Ever FDA Clearance of CRISPR/Cas13 RNA-Editing HG202 for Macular Degeneration
-
Dec 06,2024
HuidaGene Therapeutics Announced First Patient Dosed in the HERO Clinical Trial of HG204 for MECP2 Duplication Syndrome