Your privacy is very important to us. When you visit our website, please agree to the use of all cookies. For more information about personal data processing, please go to Privacy Policy.


HuidaGene's Sixteen Abstracts Highlight the Strength of the Pipeline and Platform to Advance Genetic Medicines at the 2024 ASGCT Annual Meeting
SHANGHAI and CLINTON (NJ), April 08, 2024 – HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company developing potentially curative genomic medicines, today announced its participation at the American Society of Gene & Cell Therapy (ASGCT) 27th Annual Meeting, an international forum where the latest gene and cell therapy developments are presented and critically discussed, being held May 7-11, 2024, in Baltimore, Maryland.
The Company’s central nervous system (CNS), ophthalmology, and neuro-myology programs and the technology platforms will be highlighted in 16 abstracts with 7 oral presentations at the upcoming meeting. These abstracts showcase the strength and potential of the Company’s DNA-, RNA-, and base-editing technologies to treat fatal neurological diseases and hard-to-treat retinal diseases.
“It’s rare to see an early clinical-stage biotech company present so many oral presentations at the world’s largest scientific conference in the field of gene and cell therapy. The breadth of data from 7 oral talks (breaking the results of last year’s 4 oral talks) and 9 posters we’ll be presenting at ASGCT validate our sustained commitment to advancing genetic medicines, demonstrating our innovation capability in the field of gene therapy on the international stage,” said Alvin Luk, Ph.D., MBA, CCRA, Co-Founder and Chief Executive Officer of HuidaGene. “Using our advanced gene-editing technology, we aim to bring ‘one-and-done’ treatments for patients living with fatal neurological diseases making the untreatable treatable. I look forward to co-chairing a Neurologic Diseases session at this year’s meeting.”
“With the recognition of our genetic tools and animal disease models, we currently have a total of 4 programs granted both orphan drug designation (ODD) and rare pediatric disease designation (RPDD) for each program by the U.S. FDA,” commented Xuan Yao, Ph.D., Co-Founder and President of HuidaGene. “The Company is currently actively exploring flexible collaboration models such as technology licensing and/or co-development and is eagerly looking forward to an in-depth discussion with global pharmaceutical companies. I look forward to discussing the Company’s innovation at ASGCT.”
HuidaGene’s presentations at the 27th ASGCT Annual Meeting include:
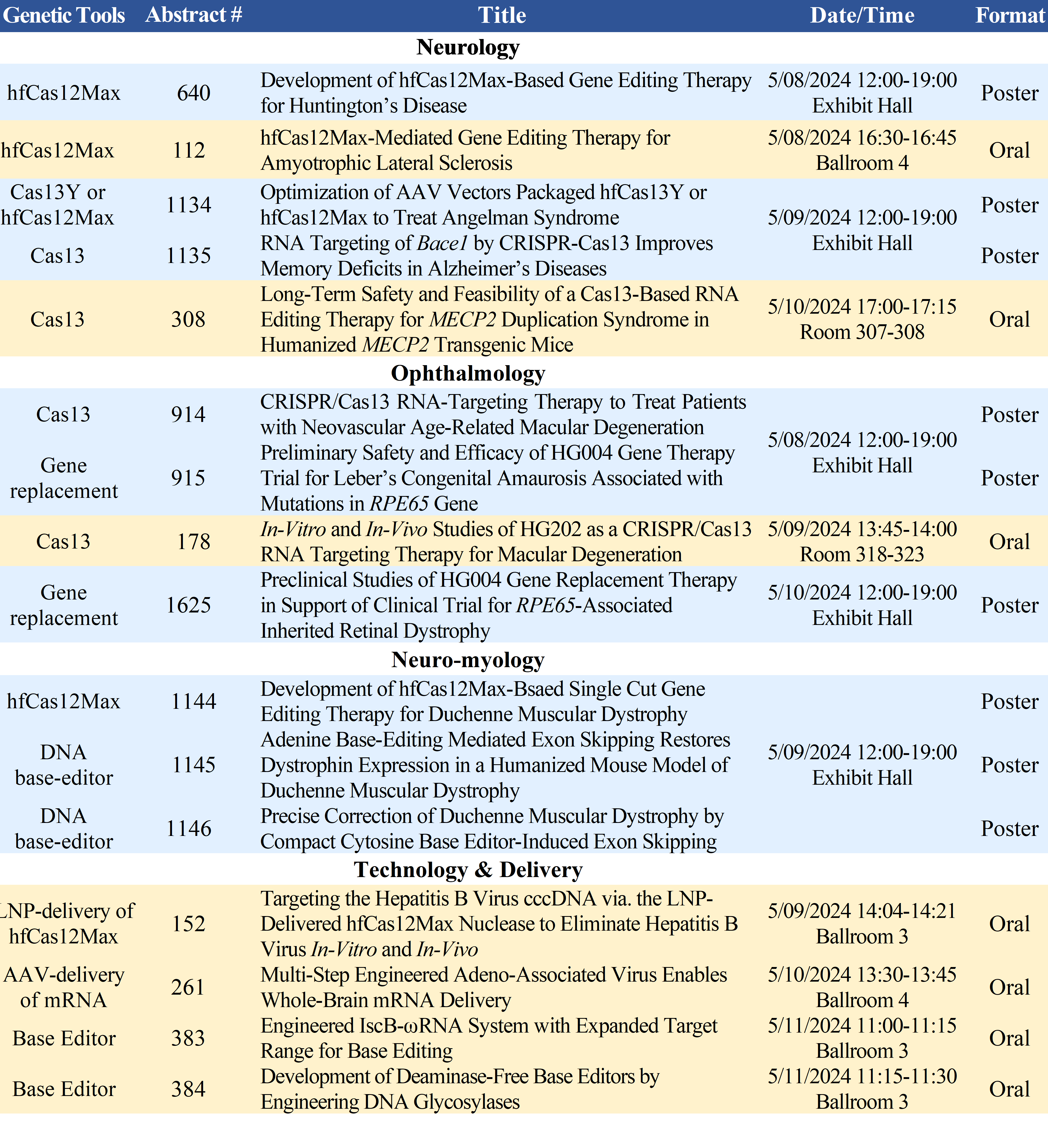
Accepted abstracts and the full preliminary program are available on the ASGCT website (https://annualmeeting.asgct.org/abstracts).
“HG-PRECISE® platform, which stands for HuidaGene – Platform for Rational Engineering of CRISPR-Cas Identification by Synergic Expertise, enables rapid discovery of Cas proteins using both AI and deep machine-learning of DNA sequencing and assembly prediction from the metagenomic database. With the independent IP rights, HG-PRECISE® generated Cas proteins (hfCas13X/Y, hfCas12Max, and ceRBE, etc) packaging into one single AAV vector have demonstrated smaller size, superior editing efficiency, and lower off-target activity in mammalian cells compared to other current widely-used Cas proteins, providing more effective, safer, and accessible genetic medicines to patients living with devastating diseases worldwide,” stated Hui Yang, Ph.D., Founder and Chief Scientific Advisor of HuidaGene.
About HuidaGene
HuidaGene utilizes its proprietary HG-PRECISE® platform to discover, engineer, and develop CRISPR-based genomic medicine. The Company is advancing clinical programs of HG004 in RPE65-associated inherited retinal disease (granted ODD and RPDD), HG202 CRISPR RNA-editing in neovascular age-related macular degeneration, and the preclinical pipeline, including HG204 CRISPR RNA-editing in neurodevelopmental disease of MECP2 duplication syndrome (granted ODD and RPDD), HG302 CRISPR DNA-editing for Duchenne muscular dystrophy (granted ODD and RPDD), and HG303 CRISPR DNA-editing for Amyotrophic Lateral Sclerosis (ALS). HuidaGene’s extensive intellectual property portfolio positions it as a leader in unleashing the full potential of genome medicines for neurology and ophthalmology. Learn more at huidagene.com or on LinkedIn.
-
Previous
FDA Awards Orphan Drug and Rare Pediatric Disease Designations to the World’s First Cas13X RNA Base-editing Therapy for the Treatment of Congenital Hearing Loss
-
Next
HuidaGene Receives Orphan Drug Designation from FDA for HG302 for the Potential Treatment of Duchenne Muscular Dystrophy after Receiving Rare Pediatric Drug Designation
recommendations
-
Dec 12,2024
HuidaGene Therapeutics Initiates M.U.S.C.L.E. Clinical Trial of HG302 for Duchenne Muscular Dystrophy and Completes First Patient Dosed
-
Apr 11,2025
HuidaGene at CRISPR MEDiCiNE 2025: A Celebration of Progress, Promise, and Patients
-
Nov 04,2024
HuidaGene Therapeutics Receives the First-Ever FDA Clearance of CRISPR/Cas13 RNA-Editing HG202 for Macular Degeneration
-
Dec 06,2024
HuidaGene Therapeutics Announced First Patient Dosed in the HERO Clinical Trial of HG204 for MECP2 Duplication Syndrome

