Your privacy is very important to us. When you visit our website, please agree to the use of all cookies. For more information about personal data processing, please go to Privacy Policy.


Why We Selected AAV as a Viral Vector for Gene Therapy?
Adeno-associated virus (AAV) is a non-enveloped, non-pathogenic virus that can be engineered to deliver DNA to target cells. Recombinant AAV vectors’ excellent safety profile, efficient transduction, and durable gene expression have emerged as the leading platform for gene delivery to various target tissues. HuidaGene’s gene replacement and genome editing therapy products deliver therapeutic genes to cells using AAV vectors.

Adeno-associated virus, AAV
*Adeno-associated virus, AAV
Advantages of Our Novel AAV
Despite tremendous progress in AAV research in the last 25 years and its continued clinical success in AAV-mediated gene therapy, there is a significant opportunity to improve the performance of the AAV platform for gene replacement, gene silencing, and gene editing and to successfully apply it to a broader range of therapeutic applications.
Our novel AAV screening platform allows us to:
-
Discover and develop novel AAV capsids with improved transduction efficacy and better safety profiles than currently available vectors.

-
Design and develop novel AAV production system to increase the yield for various AAV serotypes.

-
Develop advanced vectors specifically for genome editing.

Novel AAVs Development

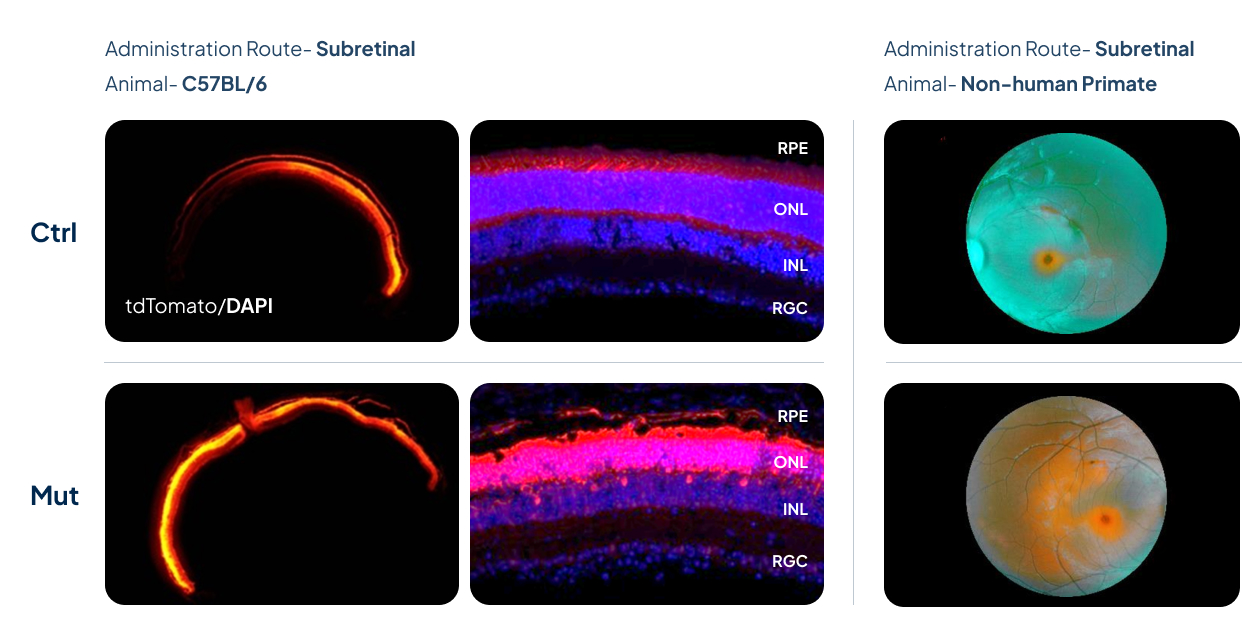
AAV Capsids Screened with Greater Infectivity of Retinal Cells
-
Control AAV (Ctrl) and capsid-engineered AAV (Mut) packaged with CAG-tdTomato were delivered into the eyes of C57BL/6 mice and non-human primates (NHPs).
-
Robust and efficient retina infection, characterized by more infected cells and infected retina layers, was observed in mut AAV group.
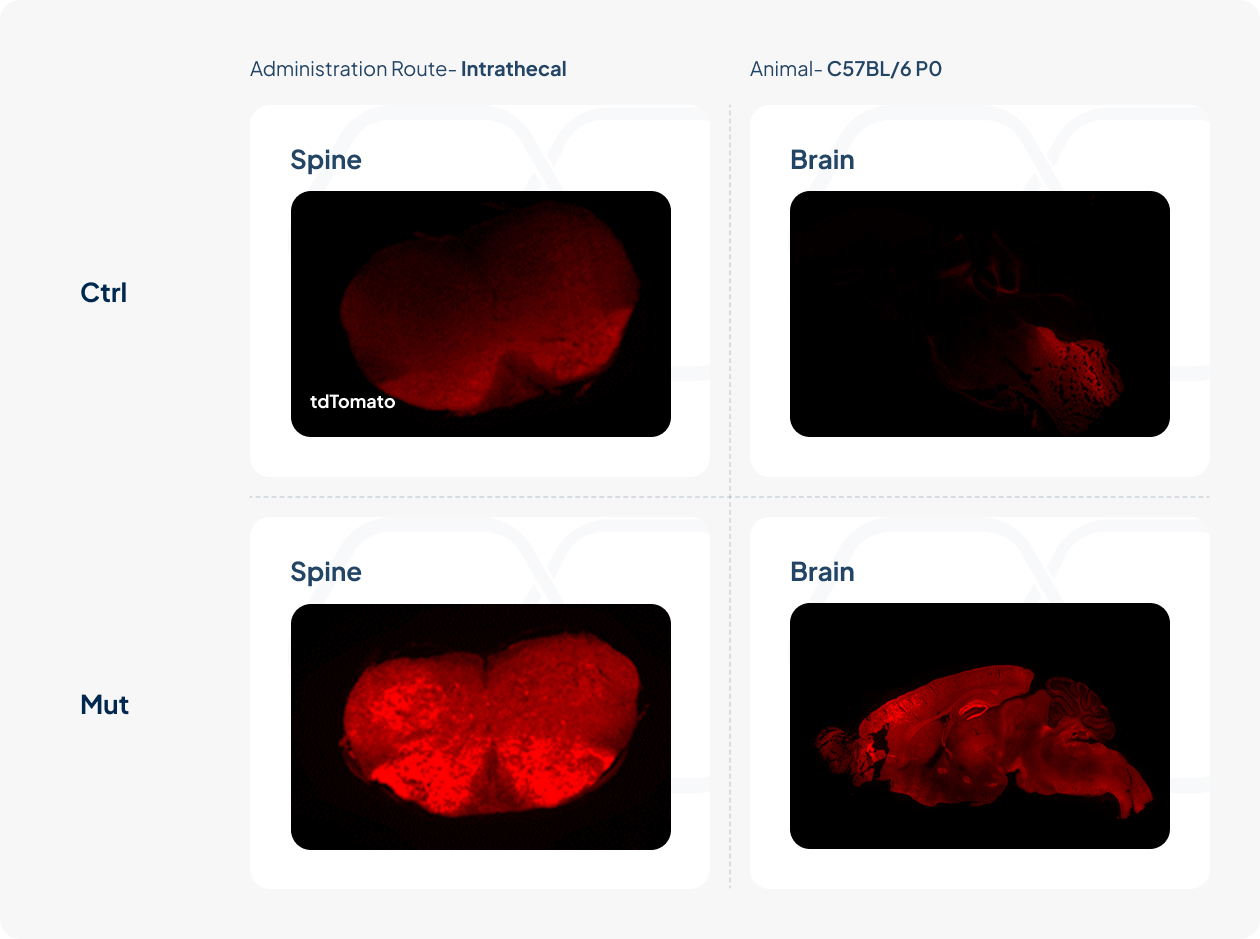
AAV Capsids Screened with Greater Infectivity of Neural System
-
Control AAV (Ctrl) and capsid-engineered AAV (Mut) packaged with CAG-tdTomato were delivered into the brain of C57BL/6 postnatal (P0) mice.
-
Efficient CNS infection, characterized by more infected neuronal cells in the spine and brain, was observed in mut AAV group.

