Your privacy is very important to us. When you visit our website, please agree to the use of all cookies. For more information about personal data processing, please go to Privacy Policy.


HuidaGene Selected for Presidential Symposium at ASGCT 2025 Annual Meeting, Showcasing 13 Abstracts Demonstrating Breakthroughs in Gene Therapy

SHANGHAI and MIDDLETOWN (DE), April 18, 2025—HuidaGene Therapeutics ("HuidaGene"), a global clinical-stage biotechnology company at the forefront of next-generation genome medicines, today announced its participation in the 28th Annual Meeting of the American Society of Gene & Cell Therapy (ASGCT), to be held May 13-17, 2025, in New Orleans, Louisiana.HuidaGene will present 13 abstracts, including one prestigious Presidential Symposium oral presentation, four additional oral presentations, and eight poster presentations, highlighting its continued leadership in applying innovative CRISPR genome-editing technologies to address severe neurological, neuromuscular, and retinal diseases.
The Presidential Symposium oral presentation will spotlight HG302, HuidaGene's CRISPR/hfCas12Max DNA-editing therapy for Duchenne muscular dystrophy (DMD). The abstract, selected among the top <1% of submissions, will be presented by Dr. Alvin Luk, CEO and Co-founder of HuidaGene, and co-authored by Professor Hui Yang, Ph.D. (Co-founder and Chief Scientific Advisor), Guoling Li, Ph.D. (Head of Neuromuscular Preclinical Research), and Yiqun Yuan, M.D., Ph.D. (Medical Lead).
"Being selected for the Presidential Symposium, one of the highest honors at ASGCT, is a remarkable milestone and affirms the transformative potential of our single-cut CRISPR genome-editing platform," said Alvin Luk, Ph.D., M.B.A., C.C.R.A, CEO and Co-founder of HuidaGene. "HG302 represents a powerful, one-and-done treatment for DMD with significant precision, safety, and durability advantages. As we advance toward global clinical translation, we see collaboration with global biopharma leaders as a key catalyst in maximizing our impact. We look forward to sharing our breakthroughs with our partners in the biopharmaceutical industry and forging new collaborations to accelerate impact."
The additional oral presentations will cover the following:
• HG004: Advancing RPE65-associated inherited retinal disease (IRD)
• HG202: CRISPR-based RNA editing for neovascular age-related macular degeneration (AMD)
• HG303: CRISPR/hfCas12Max DNA editing for amyotrophic lateral sclerosis (ALS)
• Base Editors: Development of AI-designed compact base editors for precise gene therapy

In addition, TJ Cradick, Ph.D., Chief Technology Officer of HuidaGene, has been invited to co-chair a Scientific Symposium at ASGCT 2025 titled "Advances in Genome Editing: Novel Large DNA Insertion Technologies and Their Potential Towards Curative Therapies," organized by the Genome Editing Committee. The session will highlight groundbreaking approaches in enabling large DNA insertions for in vivo applications, underscoring HuidaGene's scientific leadership in next-generation CRISPR platforms.
"Our cutting-edge CRISPR tools, including hfCas12Max, hfCas13Y, and our AI-designed base editors, bring us closer to effective, one-time treatments for devastating diseases like DMD, ALS, and MECP2 duplication syndrome," said Dr. TJ Cradick, Ph.D., Chief Technology Officer of HuidaGene, "We are proud to lead in both innovation and global accessibility, and we welcome strategic collaborations to scale impact worldwide."
HuidaGene's gene editing platforms have received broad regulatory recognition. Four of its clinical-stage programs have received Orphan Drug Designation (ODD) and/or Rare Pediatric Disease Designation (RPDD) from the U.S. FDA for a total of eight designations. Both HG004 (RPE65-IRD) and HG204 (MECP2 duplication syndrome) have also been granted Orphan Drug status from the European Medicines Agency (EMA), bringing the total number of designations for these two programs to three.
"Our proprietary HG-PRECISEÒ platform, powered by AI and deep machine learning, enables the rapid discovery and engineering of novel, compact CRISPR nucleases," commented Professor Hui Yang, Ph.D., Chief Scientific Advisor and Co-founder of HuidaGene. "By delivering high specificity and minimal off-target editing, we are opening the door to a new era of safer, more effective gene therapies for previously intractable diseases."
HuidaGene's presentations at the 28th ASGCT Annual Meeting include:
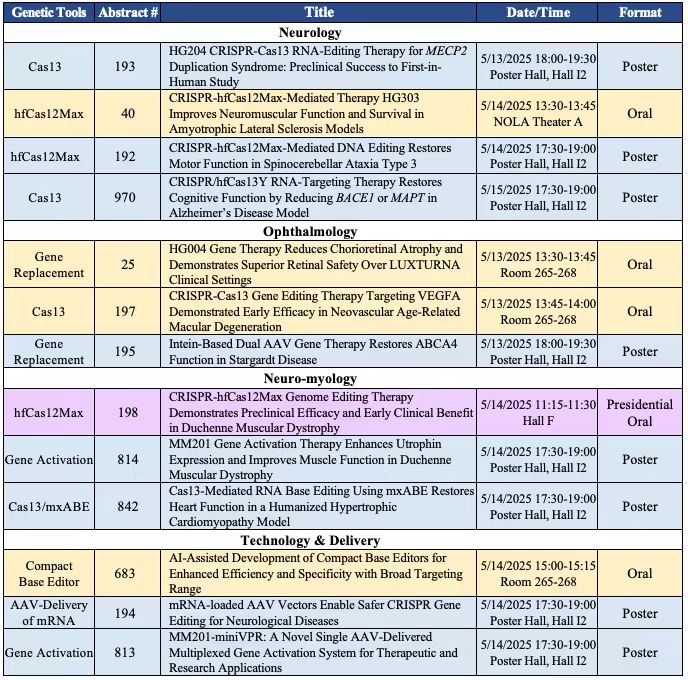
HuidaGene is actively seeking strategic collaborations with global biopharmaceutical companies to maximize the reach of its innovations. "We look forward to engaging with global biopharmaceutical companies and potential collaborators to accelerate the development and accessibility of our next-generation gene-editing therapies," added Dr. Alvin Luk. "Strategic partnerships will be essential to maximize the impact of our innovations, helping us bring these transformative treatments to patients worldwide, unlocking new opportunities for life-changing advancements. We welcome discussions with partners who share our vision to unlock the full potential of genome medicines."
About HuidaGene
HuidaGene utilizes its proprietary AI-driven, CRISPR-based HG-PRECISEÒ platform to develop potentially curative genome medicine. The company's current clinical programs include:
HG004gene replacement therapy (ODD & RPDD from the US FDA and ODD from EMA) for RPE65-associated retinal disease (IRD)
'LIGHT' first-in-human trial (NCT06088992)
China's first 'STAR' Phase 1/2 multi-national, "master protocol" clinical trial (NCT05906953)
HG202 CRISPR/RNA-editing therapy for neovascular age-related macular degeneration (AMD)
'SIGHT-I' first-in-human trial (NCT06031727)
World's first CRISPR/RNA editing therapy 'BRIGHT' Phase 1 clinical trial (NCT06623279)
HG204 RNA-editing therapy (ODD & RPDD from FDA and ODD by EMA) for MECP2 duplication syndrome
World's first CRISPR/RNA-editing 'HERO' for neurodevelopment disorders first-in-human trial (NCT06615206)
HG302 DNA-editing therapy (ODD & RPDD from FDA) for Duchenne muscular dystrophy (DMD)
World's first CRISPR/DNA-editing 'MUSCLE' first-in-human trial (NCT06594094)
The preclinical pipelines are advancing simultaneously in HG303 CRISPR/DNA-editing for ALS and CRISPR/RNA-editing therapy for Alzheimer's and Huntington's Disease. With an extensive intellectual property portfolio, HuidaGene is a leader in genome medicines for neurology and ophthalmology. Learn more at huidagene.com or on LinkedIn.
recommendations
-
Dec 12,2024
HuidaGene Therapeutics Initiates M.U.S.C.L.E. Clinical Trial of HG302 for Duchenne Muscular Dystrophy and Completes First Patient Dosed
-
Apr 11,2025
HuidaGene at CRISPR MEDiCiNE 2025: A Celebration of Progress, Promise, and Patients
-
Nov 04,2024
HuidaGene Therapeutics Receives the First-Ever FDA Clearance of CRISPR/Cas13 RNA-Editing HG202 for Macular Degeneration
-
Dec 06,2024
HuidaGene Therapeutics Announced First Patient Dosed in the HERO Clinical Trial of HG204 for MECP2 Duplication Syndrome

