Your privacy is very important to us. When you visit our website, please agree to the use of all cookies. For more information about personal data processing, please go to Privacy Policy.


About Us
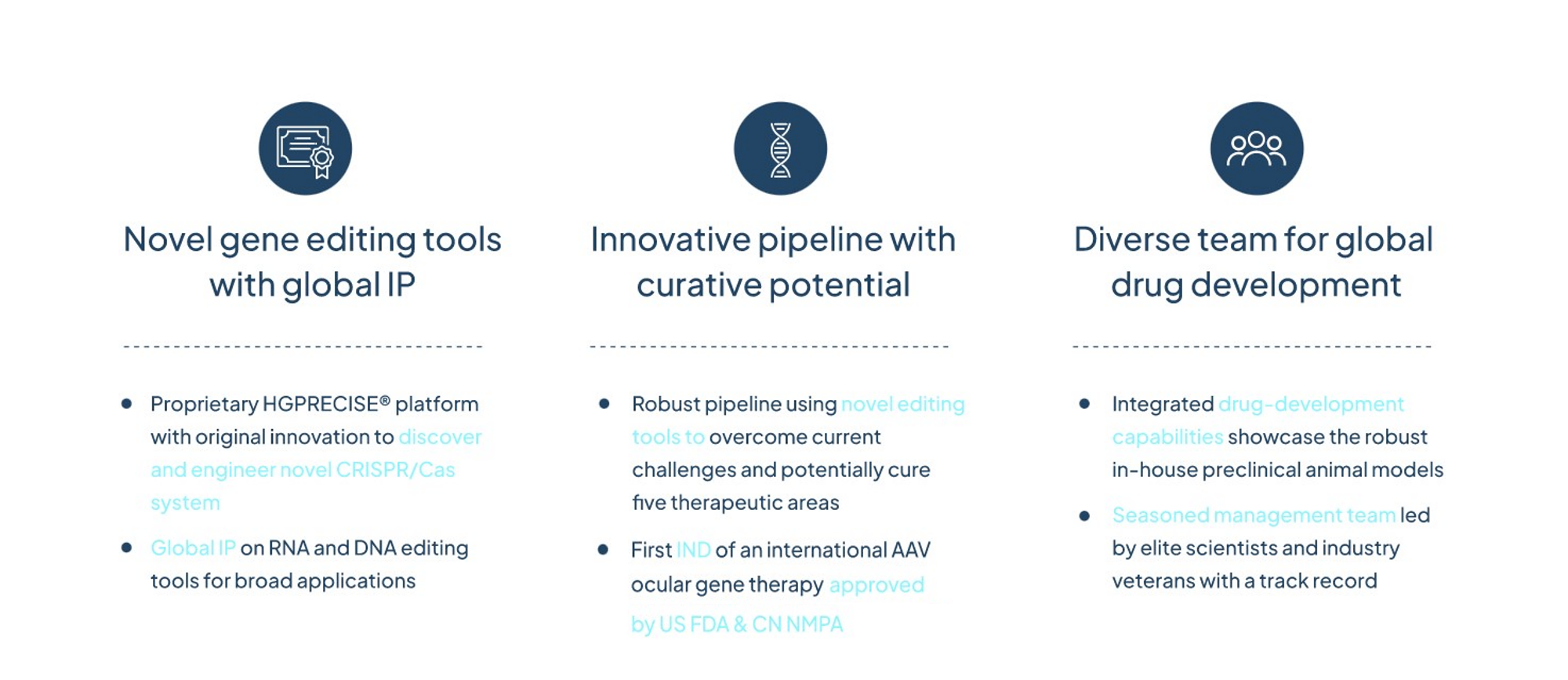
HuidaGene is a global clinical-stage biotechnology company focusing on discovering, engineering, and developing gene editing tools and gene therapies to rewrite the future of genomic medicine. Based in Shanghai and New Jersey, HuidaGene is committed to addressing patients’ needs globally with various therapeutic programs covering neurology, ophthalmology and neuromuscular. HuidaGene is committed to delivering cutting-edge gene editing tools and providing precise, safe, and effective one-and-done treatments for patients living with life-threatening conditions worldwide by repairing the cause of their disease. We are committed to transforming the future of genome-editing medicine.


Our Mission
&
Vision
Growth Footprint
-
2018
Oct. Incorporation of HuidaGene in Shanghai
Nov. Completion of Angel round of funding
-
2019
Feb. Completion of the R&D lab
Nov. Completion of Series A financing
-
2020
Nov. Completion of CMC Lab (Shanghai Waigaoqiao Free Trade Zone)
-
2021
Feb. Discovery of CRISPR-Cas13X/Y tool
May. Completion of Series B financing
Nov. Discovery of CRISPR/Cas12 tool
Dec. Completion of a first external licensing partnership with Cas13X/Y RNA editing tool
-
2022
Jan. Cas13X/Y patent granted by USPTO
Apr. Completion of Animal Facility (Shanghai Waigaoqiao Free Trade Zone)
May. Completion of Series C financing
Sept. RNA Base Editor-Cas13X patent granted by CNIPA
-
2023
Jan. IND clearance for the multi-national, master-protocol, Phase 1/2, gene therapy clinical trial of HG004 (Study HG00402; NCT05906953) to treat RPE65 mutation-associated inherited retinal diseases (RPE65-IRDs) by FDA
Jan. First patient dosed in the HG004 first-in-human (Study HG00401; NCT06088992)
Mar. HG004 gene therapy granted Orphan Drug Designation (ODD) by FDA for the treatment of inherited retinal diseases caused by RPE65 mutations
Apr. IND approval of the multi-national, master-protocol, Phase 1/2, gene therapy clinical trial of HG004 (Study HG00402; NCT05906953) by China NMPA
May. Cas12i patent granted by USPTO
May. Discovery of glycosylase-based guanine base editor (gGBE)
Aug. HG004 gene therapy granted Rare Pediatric Disease Designation (RPDD) by FDA for the treatment of inherited retinal diseases caused by RPE65 mutations
Aug. HuidaGene and Kactus announced a licensing partnership on hfCas12Max
Sep. First patient dosed with the world's first novel CRISPR/Cas13 RNA-editing therapy HG202 for the treatment of neovascular age-related macular degeneration, nAMD (Study HG20201; NCT06031727)
Oct. HG204 CRISPR/Cas13Y RNA-editing therapy granted both ODD and RPDD by the FDA for the treatment of MECP2 Duplication Syndrome (MDS)
Oct. Last Patient dosed in HG004 first-in-human study (Study HG00401; NCT06088992)
Nov. First Patient dosed in multi-national, master-protocol, Phase 1/2, gene therapy clinical trial of HG004 (Study HG00402; NCT05906953)
-
2024
Jan. HG302 CRISPR/hfCas12Max DNA-editing therapy granted both RPDD and ODD by FDA for the treatment of Duchenne Muscular Dystrophy (DMD)
Apr. World's first mini-dCas13X-mediated RNA base-editing therapy granted both ODD and RPDD by FDA for the treatment of OTOF-mutation-induced congenital hearing loss
May. HG204 CRISPR/Cas13Y RNA-editing therapy granted orphan drug application (ODA) by EMA for the treatment of MECP2 Duplication Syndrome (MDS)
May. HuidaGene and Synthego announced a licensing agreement on hfCas12Max
Jun. HuidaGene and NovoCrops entered a licensing partnership on hfCas12Max for gene editing crop development
-
2018
-
2019
-
2020
-
2021
-
2022
-
2023
-
2024

